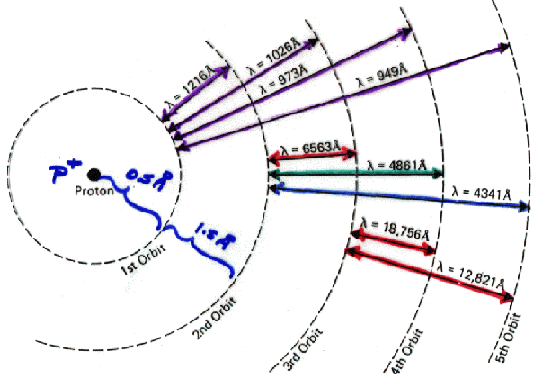
Structure of the Hydrogen Atom and Associated Spectral Wavelengths
(Thanks to Gene Smith, UCSD)
This month we will finally have a look at FUSE, the Far Ultraviolet Spectroscopic Explorer, which was launched on June 24 into a 760 km, 25° inclination, near-circular orbit on a Delta II. FUSE is a "specialty", narrowly-focussed mission, designed to study one small region of the enormous realm now open to astronomical observation, in a very detailed way. It carries four nearly identical co-aligned telescopes, each one starting with an off-axis paraboloidal primary mirror of 38.7×35.2 cm aperture and 2.24 m focal length. Each telescope feeds a high-resolution spectrometer, which cover all together the 905-1187 Å range of wavelength. Over this narrow range, FUSE has a sensitivity about 10,000 times that of the Copernicus telescope, which flew in the 1970's, and a spectral resolution of the order of 0.03Å, corresponding to a resolving power R of 24,000 to 30,000.
The FUSE wavelength range has not been well explored by previous missions. The Hubble Space Telescope's optics lose reflectivity and sensitivity at about 1150 Å, and barely overlap. FUSE covers the shortest-wavelength far-ultraviolet lines of hydrogen, the most abundant element in the Universe. Hydrogen, you may recall, has a particularly simple structure and a correspondingly simple spectrum:

Hydrogen may be in any of several energy states (quantized into discrete levels, according to quantum mechanics), corresponding to the possible orbits of its single electron. The first orbit corresponds to the lowest possible energy level, or ground state. In the absence of special circumstances, an electron in a higher orbit will generally (typically in ~10 -8 s or less) cascade down to successively lower levels, until it reaches the ground state. To conserve energy, with each downward jump it must emit radiation, a photon, with energy equal to the difference in the energy levels. Yet also according to quantum mechanics, the energy of a photon is proportional to its frequency (the proportionality constant is Planck's constant, h), and inversely proportional to its wavelength. So the light emitted must come out at one of the corresponding transition wavelengths, as shown in the diagram. All the transitions ending on the first level have wavelengths in the 911-1216 Å region: these lines are known as the Lyman series. Notice how, if the interstellar medium (ISM) were filled with pure H all in its ground state, it would be completely transparent to radiation longward of 1216Å.
Why is that? Because the photon of such radiation does not have the energy needed (about 10 eV) to lift an electron out of the ground state into any of the other possible states in which an H atom can exist. But starting at 1216Å, if a photon has just the right energy to match one of the possible transitions from the ground state to a higher orbit (wavelengths 1216Å, 1026Å, 973Å, 949Å,..., etc.) it may be absorbed. And, if it has any wavelength shorter than about 911Å (the Lyman limit) it can completely eject an electron from an H atom, ionizing it.
The cross sections for these processes are relatively very large. Thus, because hydrogen is so abundant, interstellar space becomes highly opaque at wavelengths from the Lyman limit far into the soft X-ray band. The region starting around the Lyman series is therefore ideal for the study of the interstellar medium. (It is, I suppose, obvious that if one wants to study something, say X, one really needs to choose radiation which "notices" that X is there -- perfect transparency simply won't do!)
The spectral region covered by FUSE is extremely rich even besides H. For example, O VI, oxygen with 5 of its 8 electrons missing, appears at 1032Å and 1038Å. Such highly ionized gas is only found in intensely heated regions, as in supernova remnants and other hot astrophysical plasmas. In such a violently energetic environment, H will be practically completely ionized, and so useless as a diagnostic of conditions. It is planned to use FUSE to conduct a wide survey of O VI absorption in the spectra of distant background sources to characterize the abundance and distribution of hot gas within the Galaxy.
A further major objective of FUSE is the study of the abundance and distribution of deuterium (D), or "heavy hydrogen". Deuterium is hydrogen, in which the nucleus contains one neutron in addition to the single proton that all hydrogen contains by definition. The additional mass of the nucleus due to this extra neutron, approximately double the normal value, results in a small change in the energy levels of the D atom, and a corresponding shift in the wavelengths of its spectral lines, allowing FUSE to measure its abundance relative to ordinary H. The cosmic abundance of D is of enormous fundamental importance, for the following reason.
The compelling attractiveness of the Big Bang as our theory for the beginning is largely that it explains a very great deal of what we notice about the Universe today, based on a very simple physical picture. In that picture, the Universe underwent what was essentially a free expansion, in a state of nearly thermodynamic and hence chemical equilibrium, as its temperature and density steadily dropped. Early on, in the first few seconds, the temperature was so high that atoms could not hold together, nor even atomic nuclei, nor even neutrons and protons. In that era, the Universe ought to have consisted of a reacting soup of high-density plasma, made of quarks, gluons, and the other exotic species of high energy physics. Our understanding of this period is fundamentally limited by our incomplete understanding of elementary particle physics.
After a few seconds, however, the temperature dropped to the point where the quarks combined to form protons and neutrons, yet still all reacting at a nearly uniform temperature, according to the nuclear analogs of the laws of chemistry. Shortly thereafter, as the temperature dropped further, it became possible for neutrons and protons to stick together, bound by the strong nuclear force. Yet at the same time, the neutrons, being unstable, began to decay, and after the quarks and gluons were all gone, no more n's were formed. Thus for about 1000 s, the decay lifetime of the neutron, reactions of neutrons and protons could proceed, making heavier nuclei such as D, 4He, 3He, and some little Li and Be. There were still no atoms, for it was so hot that electrons could not bind to the nuclei for more than an instant before being torn off again. And after those light elements, no nuclei could easily form, because 8Be, the next stepping stone in the chain being built up by successive addition of n's and p's, is violently unstable. So in the short time available (only a few thousand seconds before all the n's were gone) there was no way to make any heavier elements such as carbon and beyond. Much later, after about 300,000 y, the temperature dropped to the point where the electrons combined with the nuclei, and the Universe became transparent. The last light of the fireball, at a temperature of a few thousand K, survived to be red-shifted by a z factor about 1000, to become the 3°K cosmic microwave background radiation we see today.
Now it is a beautiful fact that the abundances of the light elements in the oldest stars and gas are approximately but strikingly in accord with the predictions of a this rather simple and elegant model: just react free n's and p's in a hot plasma in thermodynamic equilibrium, cooling as it expands. The agreement is good enough that we are almost irresistibly drawn to turn the argument around, assume the general physical picture is correct, and then see if we can learn more about the details of conditions in the Big Bang by the requirement of accurate agreement with the observed abundances of the light elements, of which D is especially promising. If this line of investigation were to fully succeed, we might learn clues about such things as the ratio of matter to energy in the Big Bang, deviations from perfect homogeneity, isotropy, and thermodynamic equilibrium if any, exotic components like primordial black holes, possible forms of dark matter, and the initial conditions for the formation of galaxies. Of course, to carry this program through, we must untangle the primordial D abundance from numerous nonessential complications, the worst of which is that D is a fragile and yet incendiary nucleus, easily destroyed in the interiors of stars. An extensive survey of its presence in stars and gas is needed to understand these effects and reliably estimate the primordial abundance. Such a survey is a primary objective of FUSE.
To conduct these large studies, the FUSE science team, under Principal Investigator Dr. Warren Moos of Johns Hopkins, will observe hundreds of astronomical objects, using about half of the observing time during the three-year mission. The remaining observing time is devoted to a Guest Investigator program, available for investigations proposed by astronomers world-wide. FUSE is a joint project between NASA and Johns Hopkins in collaboration with the Centre National d'Etudes Spatiales (France), the Canadian Space Agency, the University of Colorado, and the University of California, Berkeley. The spacecraft was built by Orbital Sciences Corporation, and the project was managed by the Applied Physics Lab of Johns Hopkins.
Further information is available on the web, eg, via the following links:
FUSE Home page: http://fuse.pha.jhu.edu/
Observers' Guide: http://fuse.pha.jhu.edu/support/guide/guide.html
Design and expected performance of instrumentation:
http://fuse.pha.jhu.edu/papers/technical/spie2807/2807-01.html.